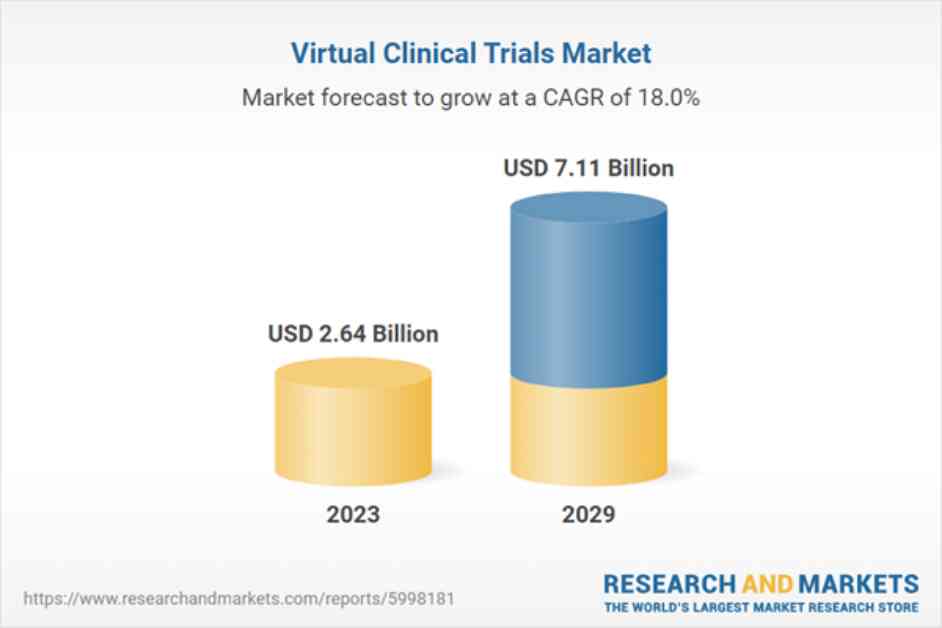
The Virtual Clinical Trials Market is experiencing significant growth in women’s health research, with the market expected to reach USD 7.11 billion by 2029. One of the key trends driving this growth is the breaking down of barriers in women’s health clinical studies. Virtual clinical trials are helping to ease restraints to participation by leveraging tools, technology, and systems to collect data remotely from patient participants. This approach is particularly beneficial in women’s health studies, where patient recruitment can be more challenging due to socioeconomic factors.
In addition to breaking down barriers, virtual clinical trials are also integrating more advanced systems and tools, such as eConsent, electronic patient-reported outcomes, and wearable devices for real-time monitoring of health parameters. The use of a bring-your-own-device (BYOD) strategy is also gaining popularity, allowing patients to share study data using their own internet-enabled devices.
Furthermore, virtual clinical trials offer a more patient-centric approach and increased participation by simplifying the process for patients from all backgrounds to participate without the need to travel long distances or be connected to a specific institution. Advanced systems under virtual clinical trials, such as Clinical Data Management (CDM) systems, help to collect and manage data securely without disrupting patient time.
Despite the growth and benefits of virtual clinical trials, there are industry restraints to consider, such as ethical considerations and regulatory compliance. Virtual clinical trials must comply with diverse regulatory requirements and ethical standards to ensure patient safety and data integrity. Ethical challenges, such as informed consent, can still be challenging in virtual clinical trials, as patients may not fully understand the risks and depth of the trial.
Segmentation insights reveal that interventional study types hold the largest market share in virtual clinical trials, with late-stage trials showing significant growth. Small and mid-sized companies dominate the market, with a focus on CNS diseases showing prominent growth in therapeutic areas. North America leads the global virtual clinical trials market, driven by advancements in clinical trials, digitalization, and IoT adoption in healthcare.
In conclusion, the Virtual Clinical Trials Market is experiencing rapid growth, particularly in women’s health research. By breaking down barriers, integrating advanced systems and tools, and offering a more patient-centric approach, virtual clinical trials are revolutionizing the way clinical studies are conducted. However, industry restraints such as ethical considerations and regulatory compliance must be addressed to ensure the continued success and growth of virtual clinical trials.