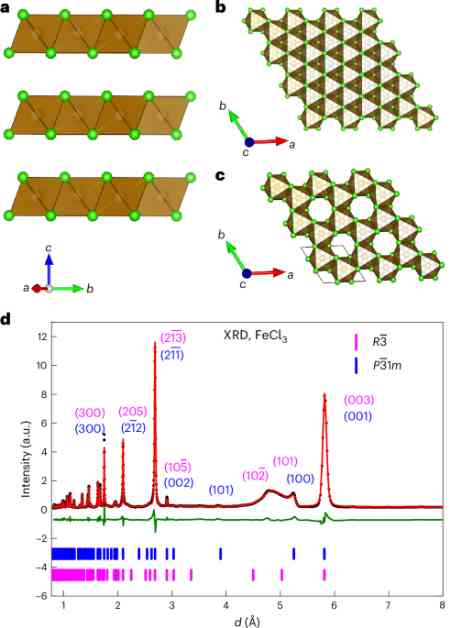
Iron trichloride (FeCl3) is emerging as a promising low-cost cathode material for solid-state lithium-ion batteries. This development is significant in the field of battery technology as it offers a cost-effective and efficient solution for energy storage.
Solid-state lithium-ion batteries are considered the future of battery development due to their higher energy density, longer cycle life, and improved safety compared to traditional lithium-ion batteries. The use of iron trichloride as a cathode material opens up new possibilities for enhancing the performance of these batteries.
Research studies have shown that iron trichloride can exhibit high energy density and reversible electrochemical reactions, making it a suitable candidate for use in lithium-ion batteries. The material properties of iron trichloride, combined with its low cost and abundance, make it an attractive option for large-scale energy storage applications.
In addition to iron trichloride, other transition metal chlorides have also been investigated for their potential use as cathode materials in lithium-ion batteries. These materials show promise in terms of high energy density and stability, further fueling the development of next-generation battery technologies.
Furthermore, advancements in electrolyte materials, such as solid halide electrolytes, have contributed to the overall improvement of solid-state lithium-ion batteries. These electrolytes offer high lithium-ion conductivity and compatibility with cathode materials like iron trichloride, leading to enhanced battery performance and safety.
Overall, the research and development efforts in the field of battery technology are driving innovations in materials and design, paving the way for more efficient and sustainable energy storage solutions. The use of low-cost iron trichloride as a cathode material for solid-state lithium-ion batteries represents a significant step forward in achieving this goal.